
First 10 Drugs Announced for Medicare Price Negotiation
On August 29, 2023, the Biden-Harris administration revealed the first 10 drugs chosen for Medicare price negotiation under the Inflation Reduction Act (IRA). Five of these drugs treat cardiovascular conditions such as atrial fibrillation and heart failure. This is a significant step towards improving access to lifesaving therapies for cardiovascular conditions that affect millions of older US residents.
The rising prevalence of cardiovascular and metabolic diseases in the elderly (65 years and above) has been a significant factor in the development of new pharmaceutical treatments. This has also caused a shift in standard care practices, with the adoption of newer, costlier brand-name drugs in place of older, more affordable generics.
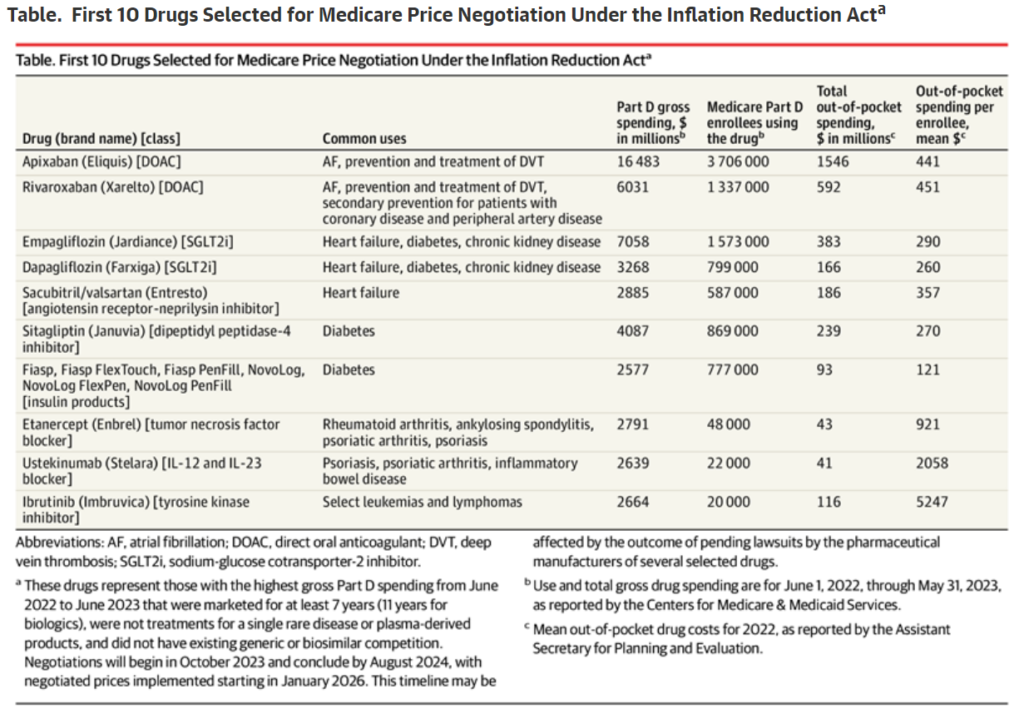
For example, the replacement of warfarin with direct oral anticoagulants (DOACs) for preventing stroke in atrial fibrillation patients is discussed. Warfarin comes at a mere $4 per month, whereas DOACs can cost between $378 to $566 monthly. This price difference has resulted in a nearly 17-fold surge in Medicare’s annual expenditure on oral anticoagulation therapy, from an estimated $440 million in 2011 to a whopping $7.4 billion in 2019.
This narrative is continued by examining the evolution of medical treatment guidelines for patients suffering from heart failure with reduced ejection fraction (HFrEF). The previous standard treatment comprised three affordable generic drugs: a β-blocker, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and a mineralocorticoid receptor antagonist. The current standard, however, includes sacubitril/valsartan and a sodium-glucose cotransporter-2 inhibitor. The cost disparity is significant. While the former regimen could cost as low as $10 a month, the current standard-of-care regimen can exceed $1200 a month.
Implications for Patients and Manufacturers
The IRA plans to address the issue of high medication costs by negotiating prices with drug manufacturers. They anticipate this negotiation will save the government $100 billion from 2026 to 2031. The lower negotiated price will also reduce out-of-pocket costs for patients. Additionally, the IRA will mandate all Part D plans to cover drugs with negotiated prices, improving access to these medications.
