Medical schemes in South Africa are prioritizing mental health as a key component of managed care services. In 2019 CMS published a PMB definition guidelines for mental health emergencies.
“It is still concerning that beneficiaries of medical schemes attracted copayments as high as five (5%) for services such as a psychiatry benefit. Prior studies have also found that medical scheme members affected by mental illness and in most cases were discriminated against by funders. There needs to be a concerted effort to reprioritise mental health,” said Willie
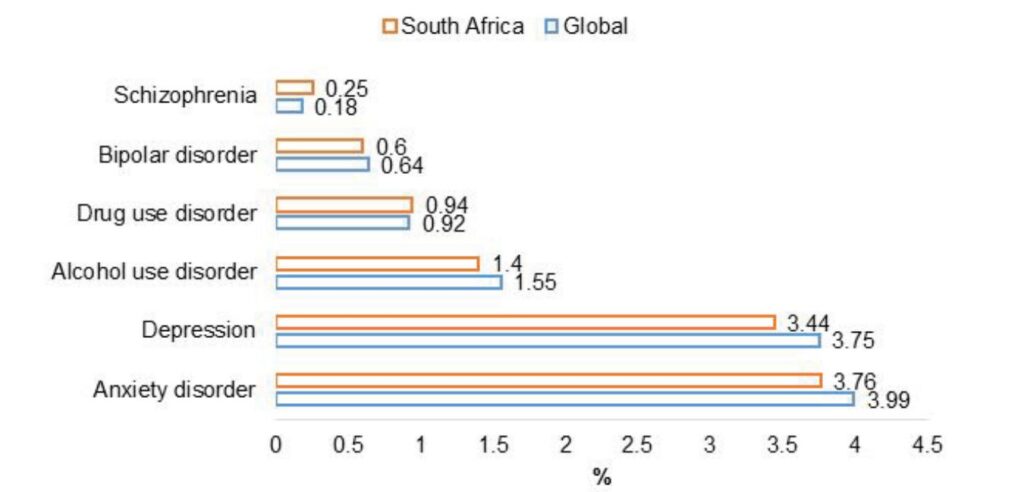
A study analysed data from 2014-2018 and found the most prevalent mental disorders in South Africa in 2017 were anxiety and depression. However, only schizophrenia and bipolar mood disorder are the only conditions included in the PMB Chronic Diseases List (CDLs).
It is worrying that mental disorders like depression and anxiety are not covered under PMBs. We need a review of PMBs to include these conditions. A designated medical scheme provider should consider resource scarcity and co-payment rules.